An Overview of CMMS Capabilities for Calibration and Maintenance Professionals in Highly Regulated Industries
Can a Computerized Maintenance Management System (CMMS) help you do your job more efficiently? As a calibration or maintenance professional in a highly regulated manufacturing organization, you are responsible for ensuring that equipment is operating correctly and safely in your facility. A CMMS can help you schedule, streamline and optimize these tasks, giving you more time to focus on preventive maintenance and minimizing equipment downtime.
A CMMS is a software system that helps to organize and automate many of the tasks associated with calibration and maintenance. This includes scheduling, tracking, and reporting on equipment, inventory, and work orders. Additionally, it can ensure compliance by providing a single location for documentation and record-keeping, which are essential in highly regulated industries.
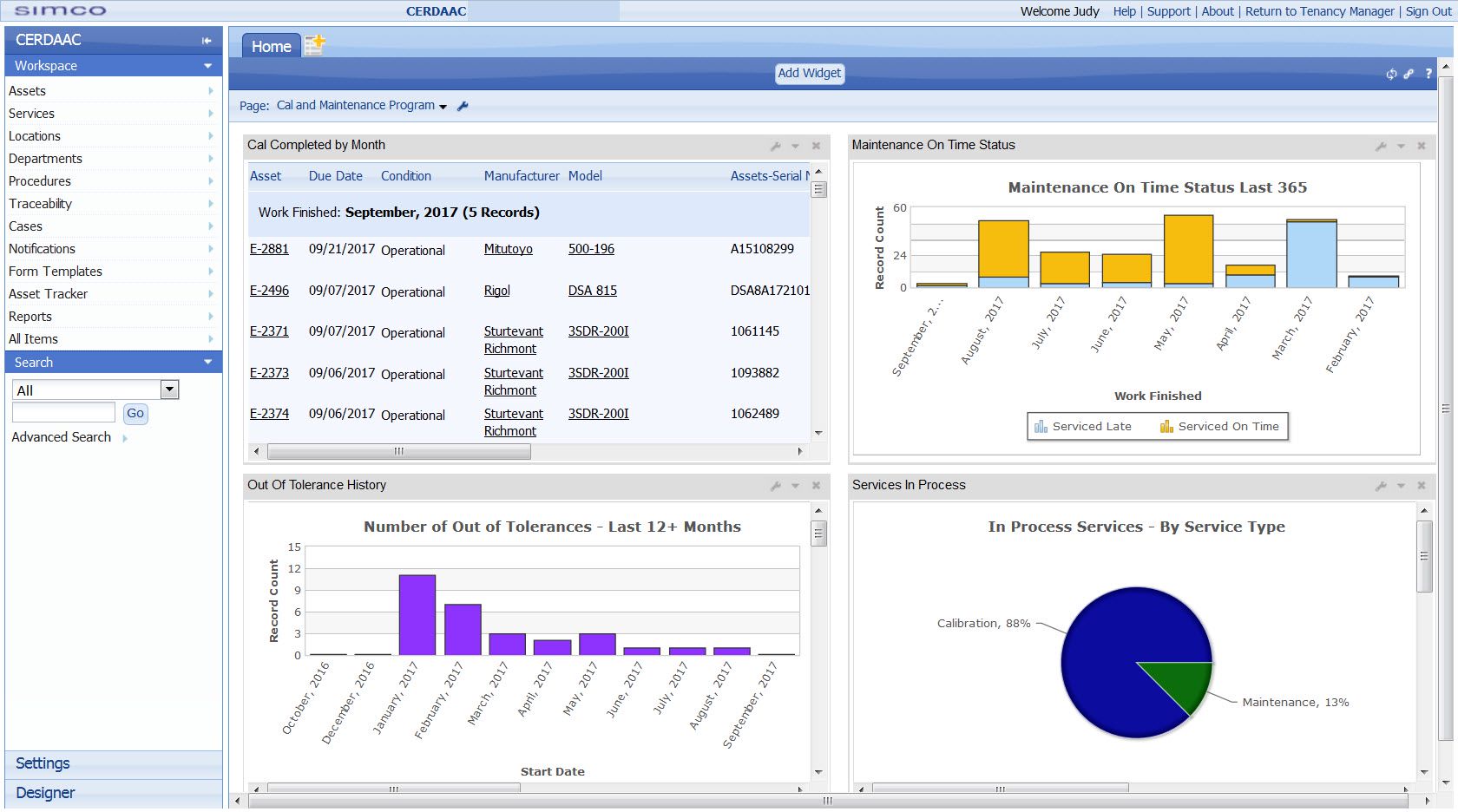
A CMMS is a software system that helps to organize and automate many of the tasks associated with calibration and maintenance.
Many organizations may look to existing software, such as enterprise resource planning (ERP) systems or Quality Management systems to provide the functionality they need for calibration and maintenance tasks. However, these systems are not built to handle the specific tasks calibration and maintenance professionals need to do their jobs more efficiently and effectively. A CMMS system is built specifically to automate these processes, giving calibration and maintenance teams the tools they need to track, maintain and manage production related equipment.
Key Features of a CMMS
Some of the key features of a CMMS include:
- Equipment tracking: Detailed equipment tracking, including the ability to schedule and track calibration and preventative maintenance.
- Inventory management: The ability to track and order replacement parts.
- Work order management: A streamlined process for creating, assigning, and tracking work orders.
- Compliance: Compliance with relevant regulations such as Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP)
- Reports and Dashboards: Generating reports on key performance indicators such as equipment downtime, maintenance costs, and compliance rates.
By using a CMMS, you can improve the efficiency and effectiveness of your calibration and maintenance program, while ensuring compliance with relevant regulations. This can help you to optimize performance, reduce costs, and improve safety and compliance in your facility.
Key Considerations When Choosing a CMMS for Highly Regulated Industries
For companies that are regulated by the FDA, such as medical device manufacturers, it is critical that you choose a CMMS that will make it easier for you to comply with industry standards such as the FDA’s CFR 21 Part 11 requirement. Choosing a CMMS that is purpose-built for highly regulated industries not only helps you ensure compliance, it ensures you are choosing a system that has been built to more rigorous requirements and tested. The following are a few questions to ask of your CMMS software provider:
- Is the CMMS Pre-validated? To meet FDA requirements, software vendors and their customers must provide evidence that their software has been thoroughly tested and meets all the necessary requirements for its intended use. A prevalidated system has already been validated by the software provider, saving you significant time and cost. This involves extensive documentation and testing of individual features, functions and use cases.
- Will the CMMS adapt as the organization’s needs change? As your needs change and your organization grows, is it easy to adapt the system to new processes and requirements? Can you easily add new solutions?
- How easy is it to configure the CMMS to my specific requirements? Every manufacturing organization is different, with its own processes and workflows. Make sure the system you select is easily configurable to your needs. Is it easy to add new, custom fields and workflows? Your CMMS should adapt to the way you work, not force you to change your processes to fit the system’s limitations.
- Is the CMMS easy to use?: does the system provide an intuitive interface? Is it easy for new users to learn? A complicated user interface can take up more time and resources to learn and limit the system’s utilization.
- Who will install, update and administer the software? What hardware is required? Modern software applications are cloud-based, making it easier for users to access anytime, anywhere, and greatly reducing IT administration requirements. That means there is no on-premise software to install. The vendor hosts the software on its own servers, is responsible for security, and provides automatic updates.
- Does the CMMS integrate with other operations systems? What systems does your CMMS need to integrate with to provide maximum value? Do you have ERP and other operational or quality systems that you use? Avoid a CMMS that will become a data silo and make it difficult to share data and insights.
These are just a few considerations when selecting a CMMS system for your regulated organization’s needs. For more details on how to choose software that meets your stringent requirements, read our white paper Is Your Quality Software Helping or Hindering Continuous Improvement.”