Knowing the Answer Can Save Significant Implementation Time and Cost
One of the most important questions calibration, maintenance and other operations professionals in FDA regulated manufacturing organizations can ask before purchasing a new software system is whether that system has been pre-validated for its intended use. Knowing the answer can save you significant time and cost when it comes to implementing the new software and accelerating its time to value.
What is Validated Software?
Medical device manufacturers regulated by the FDA are required to validate any software used in the production of the device or in the implementation of the device manufacturer’s quality system. In addition to the devices themselves, according to FDA 21 CFR Part 11, any software systems used to create, modify, and maintain electronic records, and to manage electronic signatures, are also subject to the same validation requirements. This is an important step in the development and use of software in FDA regulated industries, as the software may be used to make decisions that have significant impacts on patient safety and the effectiveness of treatments.
To meet FDA validation requirements, software vendors and their customers must provide evidence that their system has been thoroughly tested and meets all the necessary requirements for its intended use. The FDA requires these systems to be validated to ensure accuracy, reliability, and consistent intended performance, as well as the ability to identify invalid or altered records.
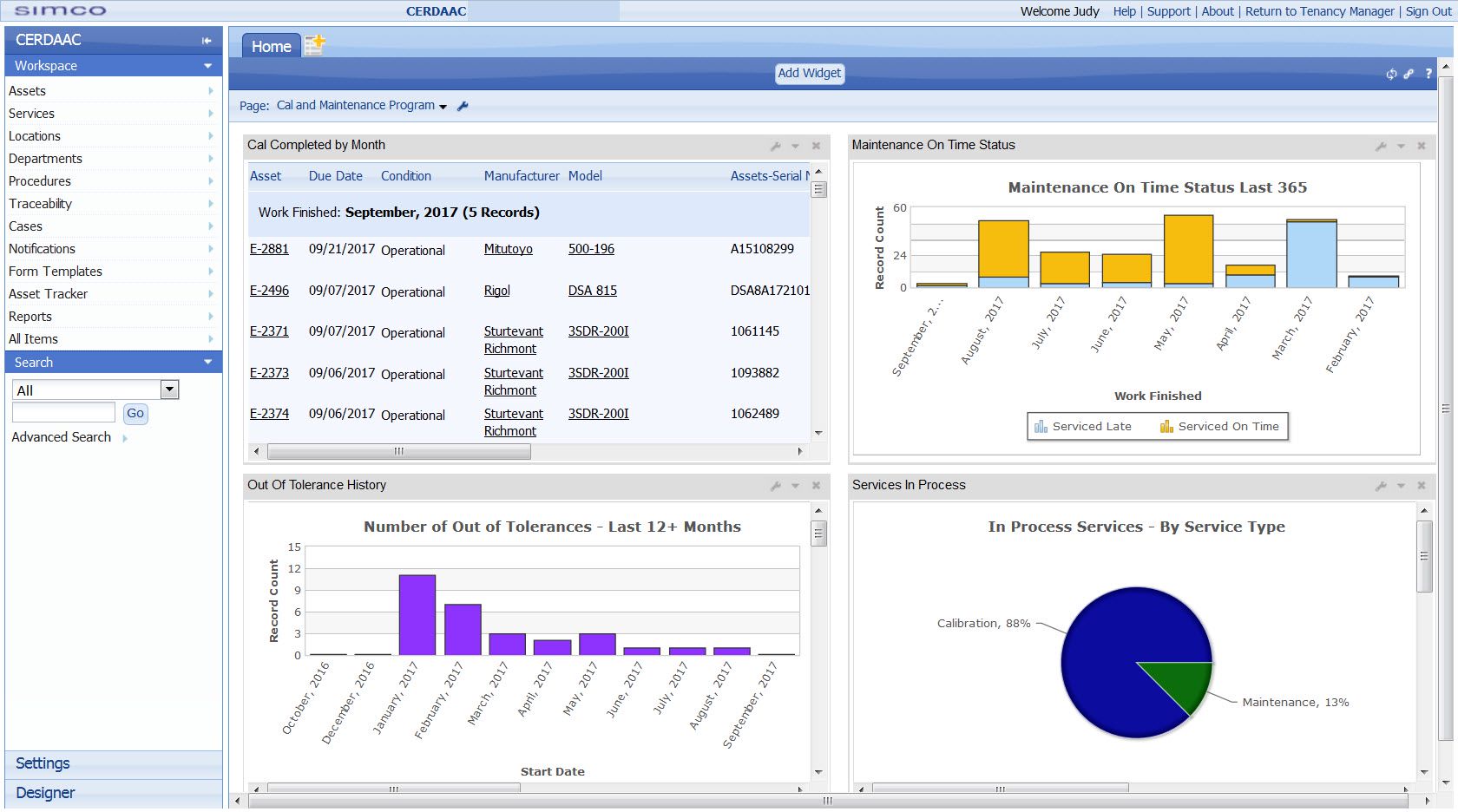
FDA regulated manufacturers that use software to track and manage services such as
asset calibration and maintenance must ensure the software is validated for its intended use.
Pre-validated vs. “Validatable” Software
Most software developers providing systems to FDA regulated manufacturers will assure you that their software meets the FDA requirements. However, there is a big difference between pre-validated software, which has been tested and certified by the software provider and/or a third-party validation firm, and “validatable” software. Validatable software means the software vendor will provide you, the customer, with documentation to help with the validation process. In other words, it’s up to the customer to perform the actual validation. This can cost organizations tens of thousands of dollars and months of IT work to initially validate the software and upon each new software release/upgrade.
In contrast, pre-validated software has already been validated by the software provider for its intended use. This involves extensive documentation and testing of individual features, functions and use cases. In order to pre-validate its software, the vendor must:
- Develop a complete definition and description of the software’s product requirements.
- Define all the potential use cases and functionality for the software’s intended use.
- Design and run a series of test cases for intended use, including workflows, adding assets, maintenance scheduling, work requests, esignatures, etc.
- Verify that the software’s features (such as “Submit Record” buttons, forms, asset records, etc.), work as intended.
- Ensure the software is tested and validated for IQ, OQ, PQ protocols:
-
- Installation Qualification (IQ) verifies that the software has been installed and configured according to the developer’s specifications or installation checklist.
- Operational Qualification (OQ) involves identifying and inspecting features that can impact final product quality.
- Performance Qualification (PQ) is the final step of qualifying the software. In this phase, the qualification and validation team verifies and documents that the user requirements are verified as being met.
On-Premise vs Cloud-based Applications
If you are using an on-premise software system, meaning one that must be installed on your company’s internal hardware and maintained by your own IT department, anytime the software is installed, changed, customized or upgraded, it must be re-validated. In fact, even if the software hasn’t changed, but is installed on a new server, it must be re-validated before it can be used, or risk violating the FDA regulation. Alternatively, cloud-based software is hosted by the software vendor on its own servers “in the cloud”, so there is no installation required at the customer site. The software is accessed by users online and no IT department support is required. In addition, upgrades to the software and/or hardware are made by the vendor and are rolled out automatically and made available to all users. For pre-validated cloud-based software, the vendor bears the responsibility of re-validating the system before an upgrade is made available.
CERDAAC Prevalidated Asset Management Cloud
CERDAAC is a cloud-based Asset Management system that is pre-validated for its intended use, saving our customers the time, expense, and liability of performing their own validation testing. To ensure CERDAAC is in compliance with FDA regulations, we work with third party validation experts who can objectively certify that CERDAAC meets the FDA’s validation requirements. In addition, because CERDAAC is a cloud-based system, every time we upgrade the software, the upgrades are made available automatically to our customers, with no additional implementation needed.
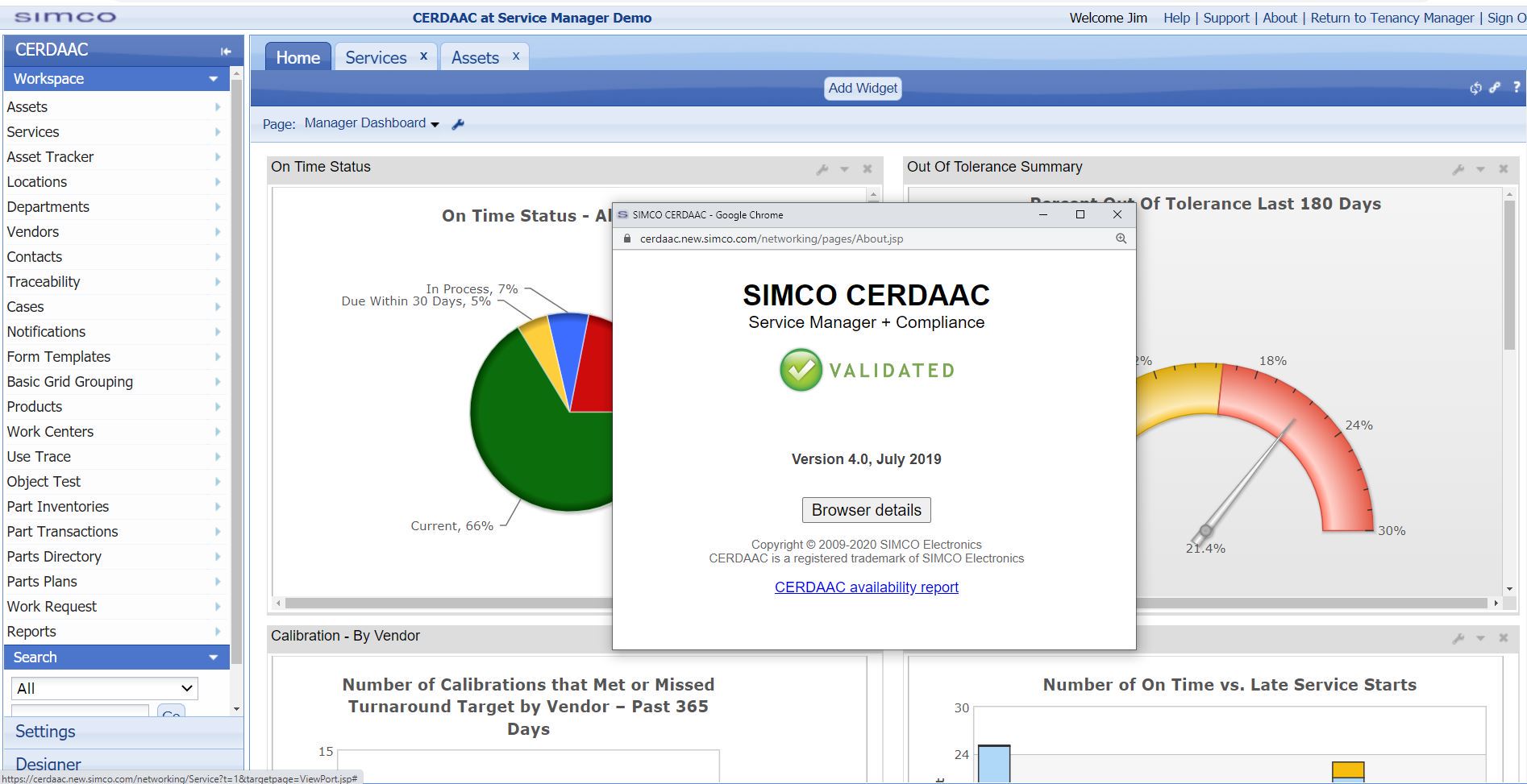
CERDAAC Asset Management Cloud is prevalidated for its intended use,
saving customers the time and cost of validating the software.
Benefits of a Pre-validated System
Even for manufacturers who are not regulated by the FDA, software validation to FDA standards is a valuable tool that ensures a system has been thoroughly tested and verified to work as intended. It is the “gold standard” by which customers can evaluate whether a system will provide the features, functionality and performance their organization needs and expects. Whether or not you are required to meet FDA standards, purchasing a pre-validated system can give you the peace of mind that the software has undergone rigorous testing and will perform as intended.
For more information about CERDAAC’s pre-validation process, talk to one of our experts at software.sales@simco.com, or Schedule a Demo.