In the intricate and highly regulated world of medical device manufacturing, facility managers face a multitude of challenges. They are tasked with ensuring the seamless operation of facilities, adhering to strict regulations, maintaining top-notch product quality, and optimizing resource management. Fortunately, Computerized Maintenance Management Systems (CMMS) have emerged as powerful allies in overcoming these challenges.
Enhanced Regulatory Compliance: Regulatory compliance is a paramount concern in the medical device industry. CMMS solutions provide facility managers with the tools to create, manage, and execute comprehensive maintenance plans. These plans can be tailored to meet the specific regulatory requirements of the industry. They allow for the scheduling of routine equipment inspections and maintenance, ensuring that critical compliance standards are consistently met and documented. These systems can generate detailed records that can be easily accessed for audits, helping facility managers stay ahead of regulatory challenges.

Real-time Monitoring and Alerts: They can integrate with sensors and automation technologies to enable real-time equipment monitoring. Facility managers receive instant alerts when issues are detected, allowing for swift action to prevent equipment failure. This proactive approach minimizes costly downtime and safeguards product quality.
Precise Equipment Maintenance: Maintaining equipment in peak condition is essential to producing high-quality medical devices. CMMSs enable facility managers to track the maintenance history of each piece of equipment. This historical data aids in predicting maintenance needs and scheduling preventive maintenance tasks. By addressing issues before they escalate, CMMSs help maintain product quality and reduce the risk of costly breakdowns.
Resource and Inventory Management: They help facility managers keep an organized inventory of spare parts and supplies, ensuring that critical components are always available. This minimizes disruptions caused by equipment failures and streamlines procurement processes, mitigating supply chain risks.
Effective Cost Management: CMMS systems provide valuable insights into maintenance expenses, helping facility managers to optimize budgets. CMMS empowers facility managers to make data-driven decisions that enhance cost-effectiveness by reducing unnecessary downtime or extending equipment lifespan through effective maintenance practices.
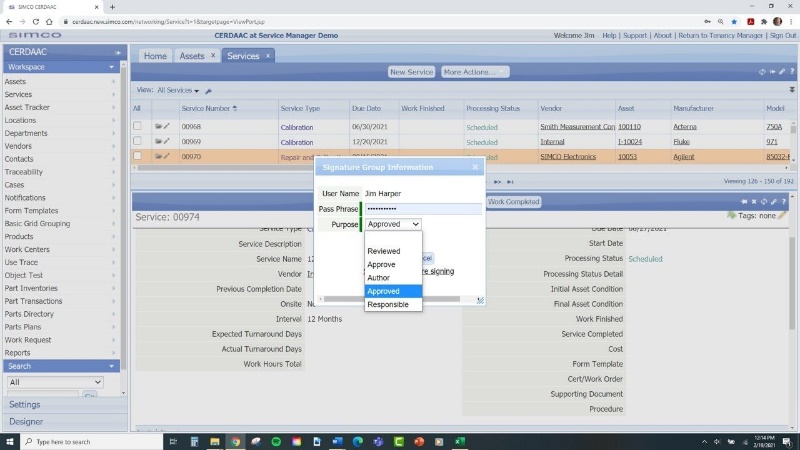
Streamlined Documentation: Medical device manufacturing requires extensive documentation of maintenance activities and compliance efforts. Automated platforms like a CMMS simplify this process by providing a centralized platform for documentation and record-keeping. This ensures that facility managers can easily access and share critical information with regulatory authorities, auditors, and stakeholders.
Adaptation to Market Dynamics: The medical device industry is subject to market fluctuations and changing demands. CMMS solutions offer valuable data and analytics capabilities, enabling facility managers to adapt to evolving market conditions. By leveraging historical maintenance data and performance trends, facility managers can make informed decisions to remain agile and competitive.
In conclusion, CMMS solutions are indispensable tools for facility managers in the medical device manufacturing industry. By harnessing its capabilities facility managers can not only meet industry demands but also drive operational excellence and ensure the production of safe and high-quality medical devices.