There are sophisticated software solutions available today that can optimize the regulated manufacturer landscape. It’s software that makes maintenance, calibration, and asset management easier, with less downtime. It ensures regulatory compliance and enhanced safety, with an incredibly attractive ROI.
Tracking, calibrating, maintaining, and managing thousands of assets, and ensuring the smooth operation of the critical infrastructure powering our modern-day existence is a huge job. And it’s one that has become unwieldy thanks to too many moving parts.
The documentation has graduated from an annoyance to a burden. And although there have been massive shifts in the way products are made, tested, shipped, and sold—asset management has remained fairly static. It’s tallied by traditional means and cannot keep pace.
Time is scarce, so we’re fast-tracking your search for software that can change all of this.
Why a CMMS is Necessary
A CMMS or Computerized Maintenance Management System is a dynamic cloud-based system of record that simplifies every aspect of asset management. It allows regulated manufacturing professionals to track, maintain, and manage production-related assets, track inventory and work orders, create reports on equipment, ensure compliance, and schedule services. Going from manual processes to a CMMS is game-changing.
This software is very different from enterprise resource planning (ERP) systems or Quality Management systems, which are significantly limited in extensibility and scope.
Our CMMS is customizable and purpose-built for calibration, maintenance, and facility teams, with all documentation and record-keeping housed in a single cloud-based location that is accessible from anywhere.
Detailing CMMS Core Capabilities
CMMS software organizes and automates critical tasks, freeing time to focus on preventive maintenance and minimizing equipment downtime. It offers proactive scheduling capabilities that improve compliance with relevant regulations and ultimately reduce CAPA findings.
As you evaluate CMMS options, these capabilities are essential:
- Detailed equipment tracking, with fields to schedule and track calibration, verification, and preventative and corrective maintenance.
- Inventory management, with the ability to track and order replacement parts.
- Supply chain management.
- Streamlined work order management that simplifies creating, assigning, and tracking.
- Monitored compliance with Good Manufacturing Practices (GMP), Good Laboratory Practices (GLP), and other relevant regulations.
- Certification, audit, and validation management, as well as non-conformance case management.
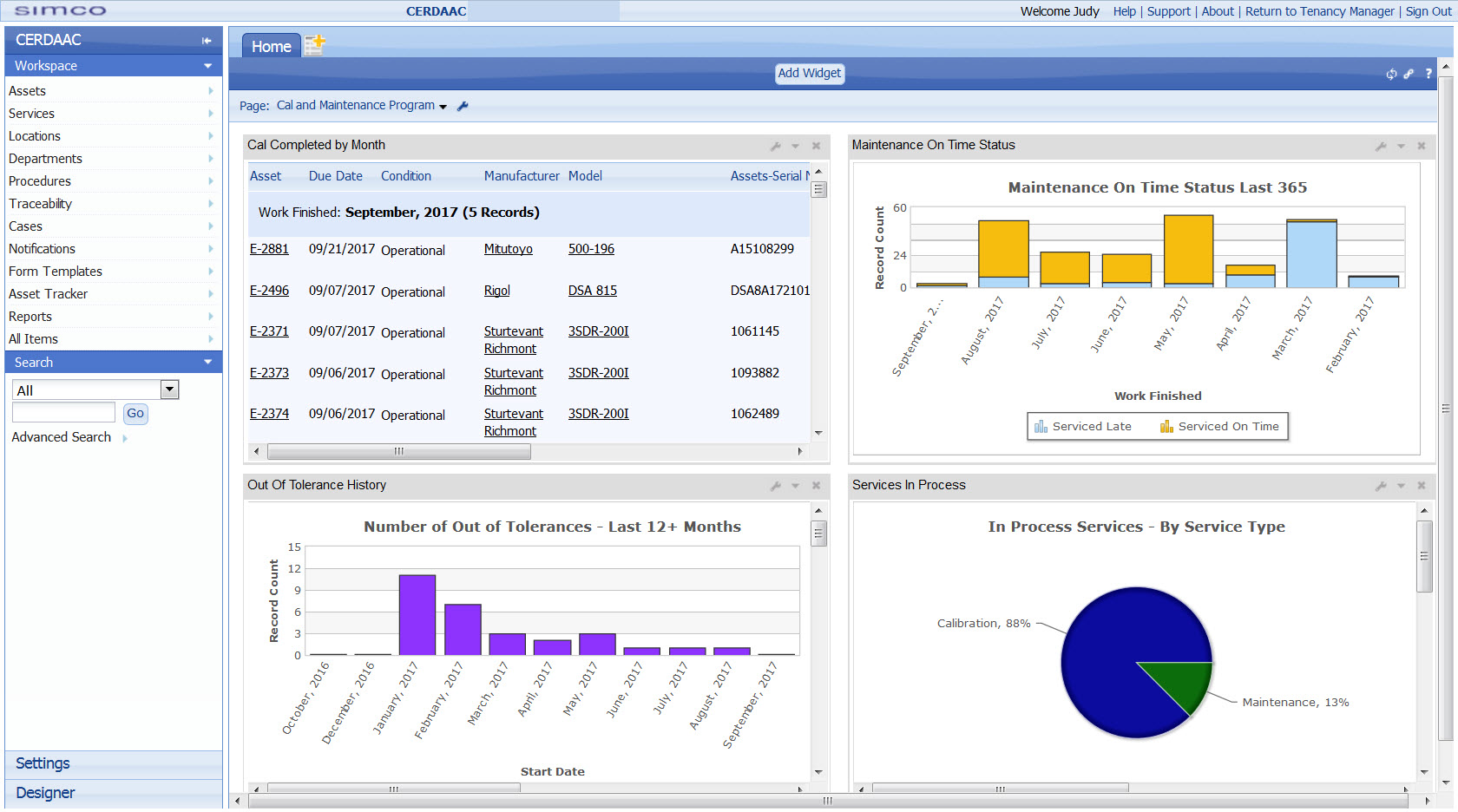
- Reports and dashboards generated that share KPIs including equipment downtime, maintenance costs, and compliance rates.
But an advanced CMMS will enhance your results on every measure with pre-validated FDA-compliant software that’s unified, audit-ready, and acts as a single source of truth. This saves regulated manufacturers significant time and cost. And that’s not all!
Not All CMMS Solutions Offer These Capabilities
Starting with the pre-validation mentioned above, many offer extensive documentation on how to validate your software, but you must complete the validation process yourself. This is time-consuming, resource-intensive, and costly work.
Native Cloud software is another key differentiator. Several software providers “cloud-wash” by claiming to offer cloud capabilities, but they’ve really just taken existing premises-based software and repackaged it as a subscription service that’s available online.
Native Cloud means it was built that way, from the ground up, and offers secure access for your users from anywhere and at any time. There is no on-premises software or hardware installation required, making the IT burden minimal. The vendor hosts and maintains the software, is responsible for security, and provides automatic updates.
Also, the premises-built solutions lack the extensibility and configurability features found in Native Cloud software. If it isn’t highly configurable, it won’t adapt to the way you work. Rather, it will force you to change your processes to fit its system limitations, which will create new unchartered territory to sort out.
An extensible CMMS supports software integration and consolidation. This streamlined approach reduces costs as it connects data so it can be shared, aggregated, and analyzed company wide. A CMMS that cannot connect will become its own data silo and create more challenges than it solves.
And it also needs to be scalable as well, to grow with you and meet your future needs. It should be simple to add new, custom fields and workflows, as well as users, teams, assets, services, partners, and sites, as needed. It should be simple to reconfigure to meet evolving needs with clicks, not code.
Having an intuitive interface and quality experts to guide you—people who understand your unique challenges —will make all the difference in the world here. Be sure to check that your provider offers solutions proven in the field and is serving companies similar to yours.
Avoiding Lengthy Deployments & Getting to Work
Adopting new software can be a gargantuan effort, and once you have everyone on board and excitement about its potential in motion, you do not want to jeopardize its implementation by losing everyone’s interest with a prolonged deployment.
Instead, search for a solution that can be implemented in a few weeks, not months or even years, which unfortunately, is a typical deployment length for many software providers.
“The deployment of CERDAAC was great. You were able to help us achieve the aggressive timeline we requested with accommodations and no hiccups. Assistance was superb, with a quick turnaround time for all questions and answers.” – Medical Healthcare Operations Company
With more than 3,000 customers and an average customer ROI of 287%, CERCAAC should be a strong contender. It adapts to new processes, requirements, and solutions as your needs evolve, which is particularly crucial in our technologically transforming landscape.

CERDAAC is a connected suite of solutions that have been developed specifically for manufacturers in highly regulated environments to manage their quality and operations. It’s a proven software suite that automates and coordinates calibration, maintenance, validation, onboarding, and other processes that surround production to ensure compliance, reduce cost, and minimize downtime.
CERDAAC’s software captures all the information you need today and anticipates and adapts for data you’ll want to keep track of tomorrow. Reach out for a demo!