Medical equipment calibration is the process of adjusting and verifying the precision of medical devices to ensure that their measurements align with established standards. This practice is not just a technical requirement—it’s a fundamental aspect of patient safety and diagnostic accuracy.
By maintaining precise, calibrated equipment, healthcare facilities uphold compliance with regulatory standards and support accurate diagnostics, directly impacting patient outcomes. Let’s explore the vital role of medical equipment calibration in patient care and share some industry-leading insight.
The Criticality of Medical Equipment Calibration
Medical equipment calibration plays a critical role in delivering accurate diagnostic results and maintaining patient trust. Inaccurate readings or performance discrepancies can lead to misdiagnoses, improper treatment plans, and potential harm to patients.
Ensuring that devices are regularly calibrated is essential to protect patients and maintain healthcare quality standards.
Calibration also ensures compliance with stringent regulations from bodies like the U.S. Food and Drug Administration (FDA) and the International Organization for Standardization (ISO). Healthcare organizations must adhere to these guidelines to avoid penalties and ensure continuous service.
Understanding the Calibration Process and Frequency Requirements
The calibration process for medical devices involves several steps:
- Initial Assessment: Evaluating the device’s current performance against manufacturer standards or relevant industry benchmarks.
- Adjustments and Verification: Fine-tuning the device to align with accuracy requirements and verifying those adjustments through testing.
- Documentation: Creating detailed reports that record the calibration process, results, and any corrective measures taken.
The frequency of calibration can vary depending on the type of equipment, its usage, and manufacturer recommendations. High-usage or critical diagnostic devices, such as those used in intensive care units, may require more frequent calibration to ensure optimal performance.
How Calibration Ensures Compliance and Diagnostic Accuracy
Regular calibration ensures that medical equipment remains within the permissible range of accuracy, as dictated by regulatory bodies and is key to maintaining regulatory compliance. This process minimizes the risk of non-compliance and related consequences, such as service interruptions and potential legal issues.
Accurate calibration also supports diagnostic precision, which is crucial for patient safety. Whether it’s blood pressure monitors, MRI machines, or infusion pumps, properly calibrated devices provide reliable data that healthcare professionals can trust. This reduces the risk of misinterpretation and promotes better treatment outcomes.
Key Features and Benefits of Medical Equipment Calibration
- Ensuring Patient Safety: Calibration ensures that medical devices produce results that meet strict accuracy standards, which in turn protects patients from misdiagnosis or incorrect treatment.
- Minimizing Errors in Diagnostic Equipment: Routine calibration helps identify potential deviations early, reducing the likelihood of significant errors that could impact patient care.
- Adhering to Regulatory Compliance: Compliance with healthcare regulations is non-negotiable, and calibration plays a pivotal role in meeting these standards.
- Preventative Maintenance and Reporting: Preventative maintenance, coupled with thorough calibration reports, keeps healthcare facilities audit-ready and ensures uninterrupted service.
- Asset Management: Effective asset management strategies, supported by calibration software, help monitor equipment status and streamline the calibration schedule.
Choosing a Solution
Tektronix, SimplerQMS and CERDAAC are three providers in the field of medical equipment calibration management. Each emphasizes precise calibration processes and regulatory compliance.
Tektronix offers specialized calibration services and has a strong presence in various technical industries, while SimplerQMS focuses on document management solutions that integrate with quality management systems for compliance.
CERDAAC stands out by providing comprehensive medical equipment calibration solutions that prioritize patient safety and regulatory adherence. Unlike some competitors, CERDAAC offers integration capabilities with other healthcare management systems, providing a seamless workflow for managing preventive maintenance, calibration, and compliance reporting. Its state-of-the-art asset calibration management helps providers stay ahead in compliance, control equipment and production downtime, and optimize operations.
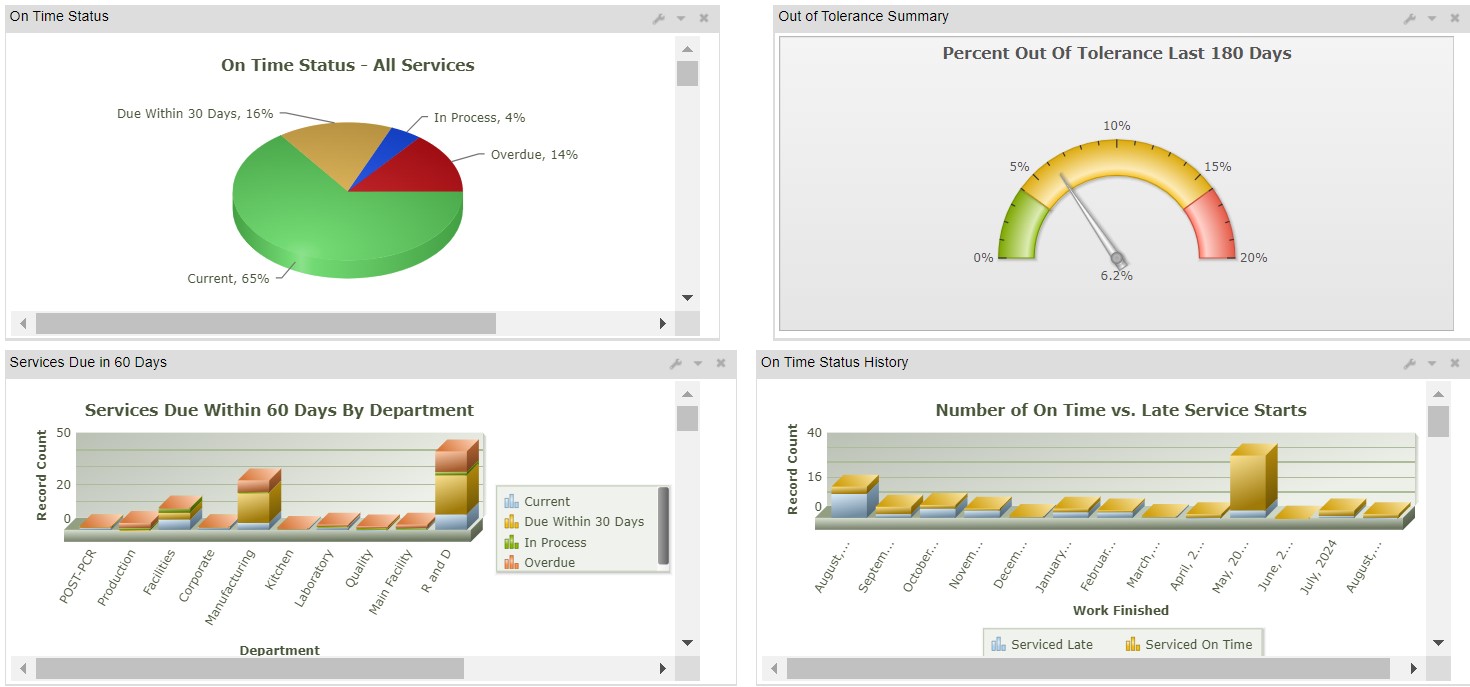
CERDAAC provides streamlined out of tolerance (OOT) management, which is an automated notification system paired with efficient case management to simplify the resolution processes, as well as in-depth and searchable audit trails.
Here is an overview of the calibration process and how each step contributes to improved patient safety and compliance.
Step 1: Initial Assessment and Equipment Check
- The calibration process begins with an initial assessment to evaluate the device’s current performance against manufacturer specifications or industry standards, ensuring that any deviations are identified early to prevent inaccurate measurements and maintain compliance.
Step 2: Calibration Setup
- The device is set up for calibration using calibrated reference instruments in a controlled environment, ensuring that conditions remain consistent to minimize errors and meet regulatory standards.
Step 3: Calibration Procedure
- The device undergoes adjustments to align its measurements with industry standards, improving diagnostic reliability and reducing patient risk while ensuring compliance with healthcare regulations.
Step 4: Verification and Validation
- The device is tested post-adjustment to confirm it meets accuracy requirements, preventing the use of inadequately calibrated equipment and reinforcing compliance and patient safety.
Step 5: Documentation and Reporting
- Detailed records of the calibration process are created, providing an audit trail for regulatory reviews and ensuring transparency that supports compliance and patient trust.
Step 6: Preventive Maintenance Integration
- Calibration often includes or triggers preventive maintenance tasks, ensuring that the device functions optimally and reducing future deviations that could impact patient safety and compliance.
Step 7: Asset Management and Scheduling
- Calibration results and schedules are logged in an asset management system like CERDAAC, automating future calibration reminders and supporting continuous regulatory compliance and equipment reliability.
Calibrations can be tailored to meet your unique healthcare management needs for centralized oversight. In an industry where patient safety is paramount, medical equipment calibration is an essential practice that healthcare facilities cannot afford to overlook. Accurate, regularly calibrated medical devices not only enhance diagnostic reliability but also ensure compliance with stringent healthcare regulations.
To explore how CERDAAC’s medical equipment calibration solutions can enhance patient safety and ensure compliance, schedule a consultation today and empower your organization with the best tools to support precision, safety, and efficiency.