Accurate calibration and metrology practices are the backbone of quality control in medical device manufacturing. These practices involve precise measurements and adjustments to ensure that equipment and instruments used in the production process meet specific standards and specifications. Here’s why they are crucial:
- Patient Safety: In the medical field, even the slightest deviation from accurate measurements can have life-threatening consequences. Accurate calibration ensures that devices like surgical instruments, diagnostic equipment, and implantable devices function as intended, safeguarding patient well-being.
- Regulatory Compliance: Medical device manufacturers must adhere to strict regulatory requirements, such as ISO 13485 and FDA regulations. Accurate calibration is essential to meet these standards and avoid costly compliance issues along with ISO 9001 and FDA 21 CFR Part 11 Compliance.
- Quality Assurance: Precise measurements and calibration maintain product consistency and reliability. This is essential to produce high-quality medical devices that healthcare professionals can trust.
- Cost Efficiency: Calibration helps identify and rectify equipment issues early, reducing the risk of production errors and costly recalls. It extends the lifespan of expensive medical manufacturing equipment.
However, calibration and metrology practices are not without their challenges:

Challenges in Calibration and Metrology for Medical Device Manufacturing
- Frequency: Medical devices often require frequent calibration and metrology checks due to their sensitivity. Managing the schedule for numerous devices can be overwhelming.
- Data Management: Keeping records of calibration and metrology data certificates, and maintenance histories for a multitude of devices can become a logistical nightmare without an efficient system in place.
- Compliance Burden: Meeting regulatory requirements means meticulous documentation and reporting. Non-compliance can result in severe consequences.
- Resource Allocation: Allocating skilled personnel and resources for calibration tasks can strain budgets and workforce availability.
How CMMS Comes to the Rescue
Implementing a CMMS in a medical device production facility can address calibration challenges effectively:
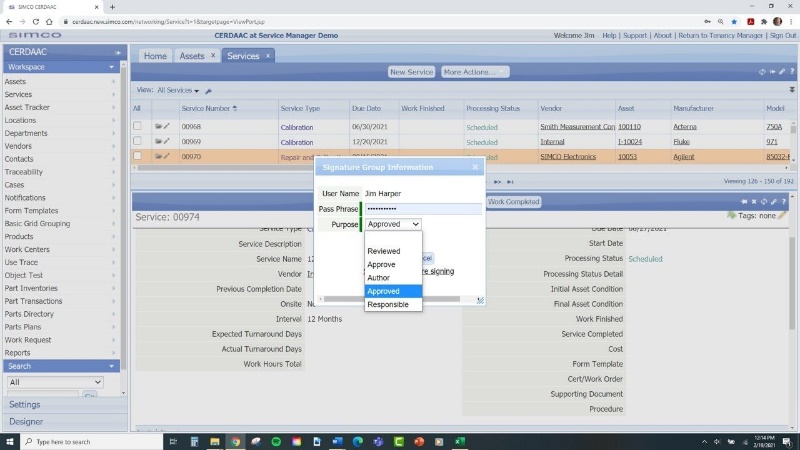
-
- Streamlined Scheduling: CMMS enables the automation and scheduling of calibration and metrology tasks. It ensures that each device receives timely attention, reducing the risk of lapses.
- Centralized Data Management: It provides the ability to store all calibration and metrology data in one accessible location. It allows for easy retrieval of certificates, maintenance history, and compliance records, simplifying audits and inspections. The data allows you to pull metrics out of the CMMS to develop reports for actionable insight in real-time.
- Resource Optimization: CMMS helps optimize resource allocation by providing insights into equipment performance. It assists in identifying underutilized or overburdened assets, enabling efficient resource allocation.
- Regulatory Compliance:
CMMS systems can generate detailed reports and notifications, ensuring that all regulatory requirements are met. This reduces the compliance burden and minimizes the risk of non-compliance issues. When completely calibrations within the system it adheres to FDA 21 Part 11 Compliance with digital signatures.
In conclusion, accurate calibration and metrology practices are paramount in medical device manufacturing to ensure patient safety, regulatory compliance, and product quality. By implementing CMMS, medical device production facilities can maintain precision and adherence to industry standards, ultimately contributing to the delivery of reliable and safe medical devices to patients worldwide.