Although there’s a big difference between a U.S. Food and Drug Administration (FDA) Form 483 Observation and an FDA Warning Letter, both are nerve-wracking notifications that manufacturers would rather avoid—and they can be avoided with a simple process shift.
The shift involves adopting a computerized maintenance management system (CMMS) that can help prevent FDA findings by keeping you many steps ahead of potential violations. When you’re carefully tracking the triggering conditions that the FDA is observing to ensure processes are on point, the likelihood of earning an observation letter significantly decreases. Correspondingly, the chance of escalation to a warning letter becomes unlikely.
What Is An FDA Form 483
During routine inspections, an FDA investigator observes several things, but it’s hard to specifically detail every potential observation.
The purpose of the form is to alert management when an investigator observes any conditions that, in their judgment, may constitute violations of the Food Drug and Cosmetic (FD&C) Act and related Acts.
Each observation noted will be clear, specific, and significant pointing to conditions or practices observed that “would indicate that any food, drug, device or cosmetic has been adulterated or is being prepared, packed, or held under conditions whereby it may become adulterated or rendered injurious to health.”
According to the FDA’s Office of Regulatory Affairs (ORA), the top five 483 citations include:
- 21 CFR 211.42(c)(10)(iv)-Environmental Monitoring System
- 21 CFR 211.113(b)-Procedures for sterile drug products
- 21 CFR 211.192-Investigations of discrepancies, failures
- 21 CFR 211.42(c)(10)(v)-Cleaning System
- 21 CFR 211.113(b)-Validation lacking for sterile drug products
Form 483 is not a final FDA determination of whether any condition is in violation of the FD&C Act or relevant regulations. However, it does create a paper trail of any potential regulatory violations found, detailing a list of deficiencies observed during the inspection. This record will include a written report, called an Establishment Inspection Report, any other evidence or documentation collected on-site, and then, importantly, any responses made by the company.
According to the FDA, there are a number of spreadsheets summarizing the areas of regulation cited, though they do not constitute a comprehensive listing of all possible observations, as some 483s are manually prepared. General observations are broken out by Product or Program Area on separate tabs of a spreadsheet under the following categories:
- Biologics
- Drugs
- Devices
- Human Tissue for Transplantation
- Radiological Health
- Parts 1240 and 1250
- Foods (includes Dietary Supplements)
- Veterinary Medicine
- Bioresearch Monitoring
- Special Requirements
- Total number of inspections and 483s
What Do Inspectors Observe Exactly?
The list of potential citations is reviewed, edited and updated on a periodic basis and there are examples of recently issued 483s on the FDA’s ORA Reading Room web page. The ORA Electronic Reading Room displays copies of ORA domestic inspection and related records. They make these records publicly available either (1) proactively at their discretion or (2) because they are “frequently requested” per the Electronic Freedom of Information Act Amendments of 1996. Some records may be redacted to remove non-public information (see 21 CFR Part 20).
Typically, observations are listed on the form in order of significance by the investigator. The observation starts with the inspector citing the law, regulation, or Act in question and then details specific conditions observed during the inspection. An example observation follows:

Description: A diagram of the information contained in a Form 483 Observation that could be made by an FDA inspector.
As noted on the FDA website, the “laws and regulations are frequently complex, identifying multiple areas for concern, [so] the short description identifies the specific element which is objectionable for an observation. The long description and reference number provide context for a short description that is cited in an observation.”
Understanding The Observations & Crafting a Response
There may be many citations for each Code of Federal Regulations (CFR) reference, addressing specific and distinct elements of the referenced law or Act. And after the observations are recorded, the review with management comes next.
The FDA ORA inspector(s) will review and discuss their findings with the senior management of the company, and it’s essential to understand each finding as the company needs to address each one in a timely manner. Beyond the issues listed, if there are others discovered during the remediation process, it is expected that the company will fix those issues as well.
A response addressing each concern should be sent to the FDA within 15 days. It should detail any relevant background information, a root cause analysis, and corrective actions taken, including a timeline for actions that are still in the process, along with a commitment to report back once they are complete. There should also be preventative actions taken and detailed in the response.
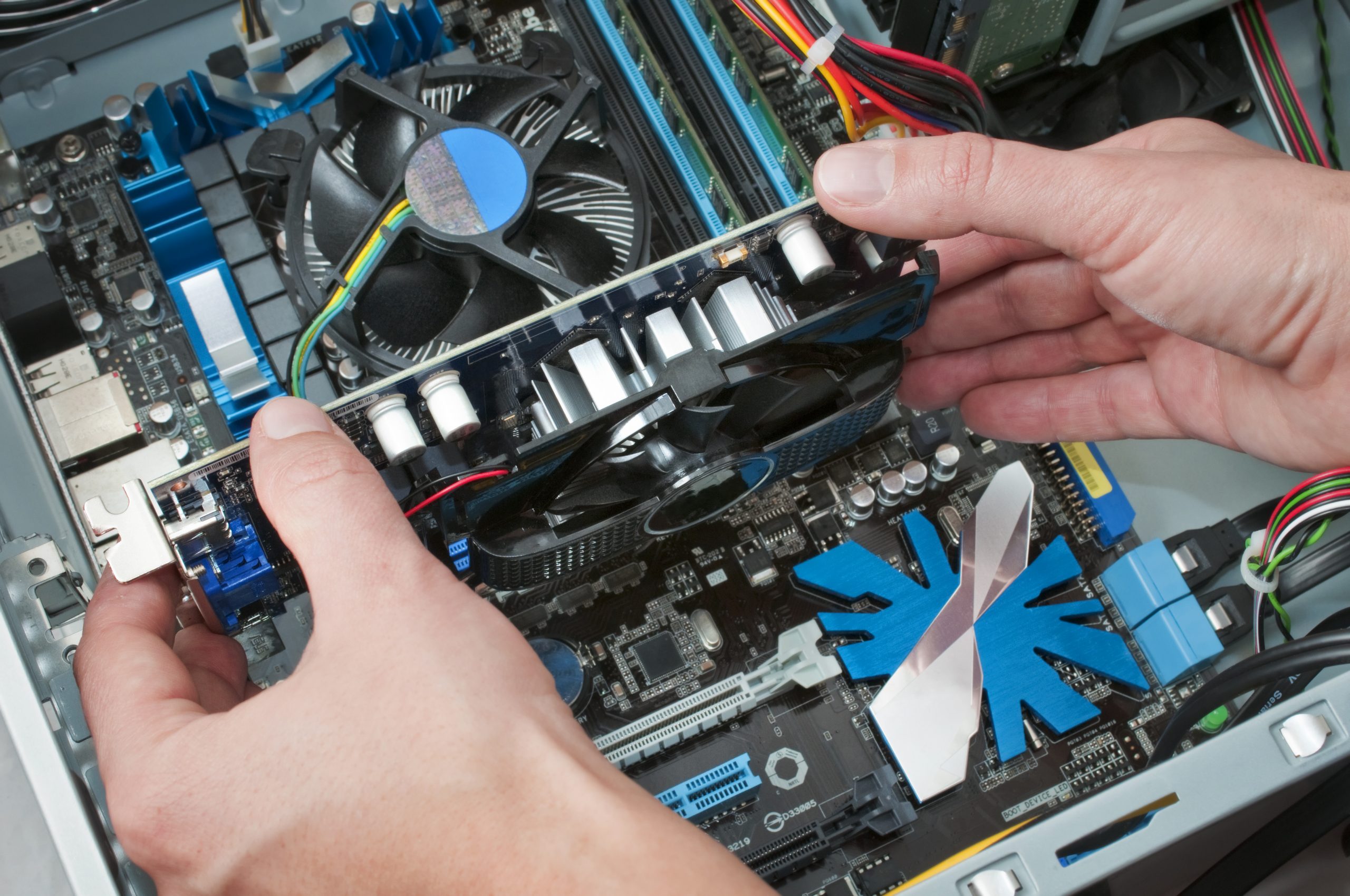
Getting any part of this wrong could result in an FDA Warning letter, which is much worse
FDA Warning Letters
If an FDA inspector observes a major compliance deficiency or just a more serious regulatory violation, the company will receive a Warning Letter instead of Form 483. A Warning Letter is also sent as an escalation to Form 483 when the company has not addressed the issue or responded within the expected 15-day window.
A Warning Letter does not always have the same 15-day timeline attached to it, as the severity of the issue may require immediate action. These letters are usually hand-delivered.
More specifically, according to the FDA, “the Warning Letter identifies the violation, such as poor manufacturing practices, problems with claims for what a product can do, or incorrect directions for use. The letter also makes clear that the company must correct the problem and provides directions and a timeframe for the company to inform FDA of its plans for correction. FDA then checks to ensure that the company’s corrections are adequate. Matters described in FDA warning letters may have been subject to subsequent interaction between FDA and the recipient of the letter that may have changed the regulatory status of the issues discussed in the letter.”
The company must address the regulatory violation before it can resume operations and bring its products to market. Working closely with the FDA to create and implement a clear plan is incredibly important at this point. Ideally, manufacturers would never experience this scenario by staying ahead of potential issues with an organized, automated approach.
Preparing Ahead of FDA Inspectors’ Unplanned Visits
Although most FDA inspections are planned well in advance, the FDA can and will show up anytime, announced or unannounced, and it’s important to always be prepared for a potential inspection.
Most potential issues in the inspectional observation data set list for regulated manufacturers can be avoided by using a CMMS with alerts and timelines that will keep you in regulatory compliance without adding to your managers’ workloads. A properly configured CMMS saves everyone time and trouble, especially when it’s specifically designed for regulated manufacturers.
Reach out for a demo and see why over 3,000 customers worldwide rely on CERDAAC, including 16 of the top 20 global biomedical companies and 14 of the top 20 global aerospace and defense companies.