- Why CERDAACWhy CERDAAC
- ProductsProducts
- SolutionsSolutions
- ResourcesResources
- CustomersCustomers
- CompanyCompany
Industries
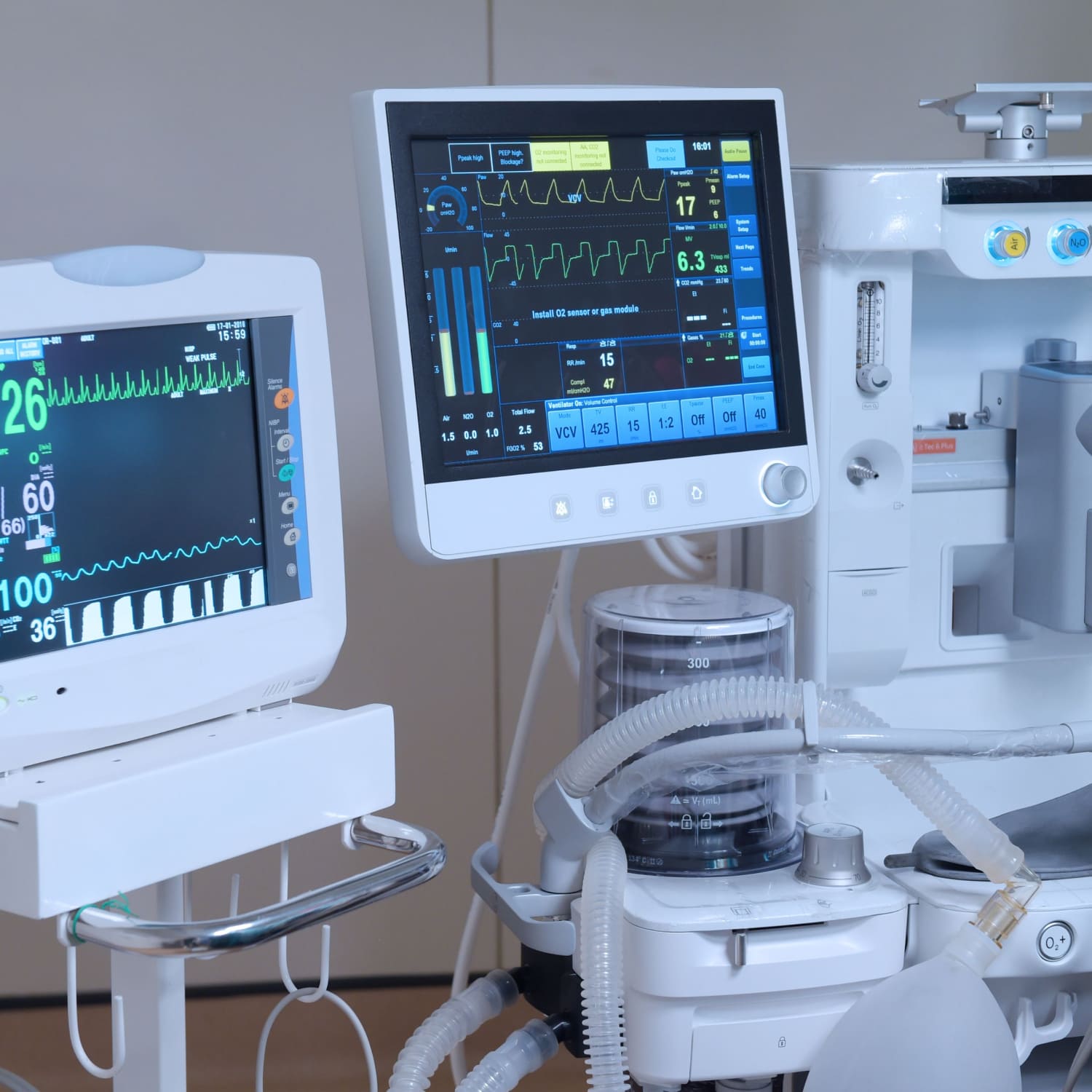
Empowering Better equipment Maintenance and Compliance Outcomes

At the heart of medical device manufacturing lies the quintessential need for reliability and compliance. CERDAAC understands this imperative and delivers a robust solution for centralizing all asset information, simplifying your audit processes, and ensuring decreased audit headaches.
CERDAAC is engineered to keep you audit-ready. Schedule preventive maintenance tasks effortlessly, track critical assets with precision, and transform maintenance management to optimize operations. Stay highly competitive and easily maintain all aspects of regulatory compliance with CERDAAC powering your enterprise.
Organize your maintenance, calibration, and operations management by orchestrating people, supplies, and processes efficiently. Drive cost savings, resource maximization, and meet manufacturing targets through efficient planning, coordination, and resource allocation.
Adopt proactive maintenance strategies tailored to your company’s specific needs. Avoid emergency expenses by forecasting maintenance requirements, ensuring timely repairs, minimizing equipment failures, and maximizing asset lifespan with CERDAAC.
Leverage CERDAAC’s advanced tracking capabilities for assured availability of essential parts and materials. Experience complete visibility and control over inventory management and work orders to ensure uninterrupted operations, reduce downtime, and optimize inventory expenditure.
Generate concise real-time reports on asset performance and maintenance history with CERDAAC. Realize the full potential of visibility across all asset types while complying with regulatory requirements effortlessly, and make proactive decisions with centralized insights into maintenance activities and compliance statuses.
Designed in adherence to FDA regulations, CERDAAC incorporates workflow automation, electronic signatures, robust security controls, and detailed audit trails. Drive productivity and current Good Manufacturing Practices (cGMP) with reduced paperwork and time-based scheduling.
Experience CERDAAC’s transformational impact on your maintenance management. Simplify the creation of maintenance schedules and ad-hoc work orders, streamline workflows, reduce scrap and error rates, and harness the power of efficient, data-driven manufacturing.
Contact us today to learn how CERDAAC can redefine your asset management experience.